Ammonia Test
Easily measure Ammonia in Manures and Composts
The Solvita Ammonia Test gives farmers, composters and laboratories the capability to readily measure volatile ammonia (NH3) present in manures, fertilizers and composts.
Ammonia escapes from manure and compost as a colorless and noxious gas. Solvita readily quantifies the presence and magnitude.
Reasons for Testing Volatile Ammonia:
1) Ammonia is a major pathway for nitrogen loss from composts and manures and measuring it can lead to improved N-content in the products.
2) Ammonia is a noxious agent in animal facilities and buildings and testing can help reduce harmful effects on animals and workers.
3) Residual ammonia in compost products poses a threat for plant seedlings growth and can be readily managed after test results by Solvita®.
How does Solvita® work?
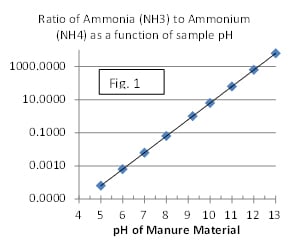
Solvita® Ammonia is a one-of-a-kind test able to distinguish free ammonia (NH3) from non-volatile ammonium (NH4) and show it visually. The test is effective over a very wide range of ammonia levels. Ammonia is related to sister compound ammonium by pH factors (see Fig. 1). Solvita successfully exploits this to distinguish the two compounds from each other.
How is Solvita® ammonia different from other tests?
Most lab methods that determine “ammonium” (NH4) (often incorrectly referred to as “ammonia” – NH3) are actually unable to distinguish which form is present since common extraction processes convert NH3 into ammonium. Solvita® distinguishes the free radical distinct from non-volatile ammonium ion. Because of the way Solvita detects the volatile NH3 there are virtually no interference in the test procedure.
Why measure ammonia separately from extractable and exchangeable NH4?
As stated above, ammonia possesses unique and strong properties that influence management practice in relation to:
- Animal intoxication
- Nitrogen losses
- Potential toxicity to plants (“phytotoxicity”)
What is Ammonia?
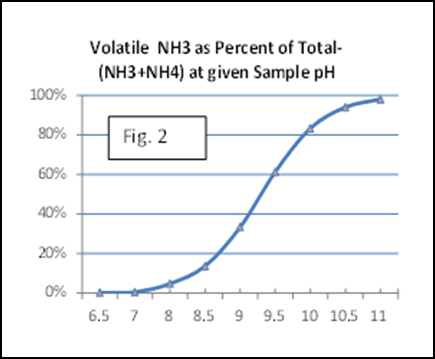
Ammonia in nature is a breakdown product of protein and urea (CH4N2O). It forms readily in freshly voided urine and feces, but also arises wherever significant forms of protein and amines are presenting in decaying organic matter or from fertilizer. Manure and soil bacteria possess the enzyme urease, which catalyzes conversion of urea into ammonia and a carbon dioxide molecule. Ammonia dissolves in the presence of water to form ammonium as follows: NH3 + H2O = NH4 + OH. The relative quantity of each species will be dictated by the pH of the substrate and is based on the pK of NH3, which is 9.3 (Fig 2.)